
In our first article discussing timing in the context of designing a new medical device, we discussed the technical feasibility process (TFP), or the stage in which medical device concepts are designed prior to the initiation of the formal design control process. The TFP has long been a subject seldomly discussed but is a critical stage of the overall design process and when carried out inefficiently or inadequately can lead to significant challenges downstream.
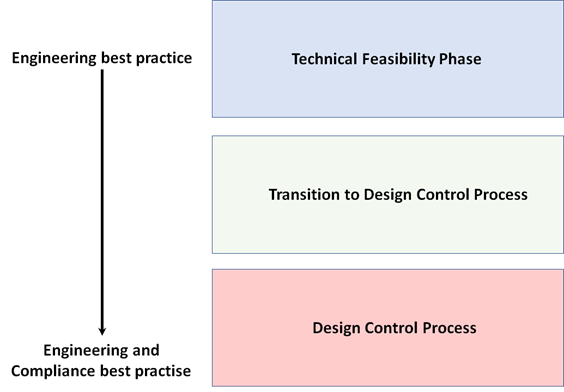
As we continue this series of articles, we want to again call attention to best practices and the importance of “timing”. Figure 1 illustrates our contention that best practices are initially dominated by engineering best practices but begin to include compliance best practices as the project progresses. Additionally, properly timing when to follow specific quality procedures can help improve the efficiency and overall success of a project. Each new article in this series will present PLT’s recommendations for when a team should consider implementing a specific quality procedure.
In Figure 1 we present three segments of the device design process. We use these segments to support our discussion on the timing of quality procedure compliance along with additional strategies/recommended best practices to support these activities:
1) During the TFP
2) During the transition from TFP to the Design Control process, and
3) During the Design Control process
This series of articles will discuss salient procedures individually in the order in which PLT recommends beginning to fully comply with throughout the design process.
Figure 1: Overview of design process stages from TFP to initiation of Design Control process
QMS introduction
An important question to answer before we get started is “What is a Quality Management System (QMS)” and “why are they helpful?” A QMS is a set of procedures that an organization establishes to provide staff with guidance on how to accomplish a certain task or group of tasks. They are not unique to the medical device industry, however a medical device manufacturer’s QMS must comply with all regional medical device specific regulations in which their device(s) is/are sold. For example, manufacturers of medical devices sold in the United States must have QMS procedures that comply with Title 21, subchapter H, part 820.
A medical device manufacturer’s QMS is analogous to a compass. It aligns a manufacturer with the relevant regional regulations (their north star) to support compliance. Further, the QMS provides alignment for staff with these regulations, setting employees on a course for executing activities and operating within a compliant environment for the entire life-cycle of all products, and the life of the organization. A challenge for employers is ensuring all employees understand and properly follow these procedures, and their procedures are regularly updated to remain current.
What are Document Controls and where do they fit?
Document controls are procedures medical device manufacturers establish to ensure all documents and records produced internally are controlled, maintained, reviewed, and approved in compliance with regional regulatory requirements. They are also used to control how changes are made to any documents, ensuring changes are documented, reviewed, approved, communicated, implemented appropriately with obsolete documents removed from use and properly maintained and controlled. Last, document controls also ensure proper version controls are implemented to help track document and device history by providing a method for traceability.
To fully appreciate the importance of document controls, PLT often presents this topic in the context of documents used to manage the life cycle of a medical device, as shown in Figure 2. As some readers may already know, the Design History File (DHF), Device Master Record (DMR) and the Device History Record (DHR) are important collections of information that outline the entire history of a device design, the comprehensive description of a specific device design version, and the summary of a specific device lot manufactured for sale, respectively. The Quality System Record consists of procedures and the documentation of activities required to manufacture a specific medical device. Where Document Controls fit in this process, and what makes them so important, is that they are the pivotal element that ensures these other components properly perform their roles. As one can see, without document controls, and more specifically version controls, manufacturers would be unable to summarize the history of their design, to create a specific version of the device for manufacture or generate records describing the specific lot of devices manufactured, or the specific revision of processes followed to do so.
Document controls, and version controls, also provide a mechanism for manufacturers to review and update internal procedures as needed to improve their processes and further ensure the safety and efficacy of their products as the product evolves.
Figure 2: The relationship between documents used to manage the life cycle of a medical device per FDA regulations

TFP QMS implementation – Document and Version Controls
As mentioned when previously discussing the TFP and timing, it is PLT’s recommendation that manufacturers begin generating documentation describing their design, with versions, as early as possible in the TFP without overtaxing the project team. Quickly creating minimally detailed test reports and design documentation is ideal for the early stages prototype development. We recommended limiting these early reports to a purpose, results and conclusions/next steps as a design/prototype evolves. How exactly this was to be done, or whether a specific procedure should be followed, and when an organization should start implementing/following those procedures, was not precisely described by PLT previously.
In the context of proper timing, it is PLT’s recommendation that during the TFP manufacturer’s first comply with purchasing controls and acceptance activities, as stated in our article discussing purchasing controls. Once a manufacturer begins generating evidence with prototypes of sufficient quality and are approaching the end of the TFP, they should begin complying with formal document and version control procedures. This ensures manufacturers are formally tracking design iterations as well as documented evidence of the design’s performance.
PLT insists that its crucial to strictly follow document control procedures for the final design out of TFP. This provides a formal framework to support staff and ensures design documentation and test results are properly documented at this critical stage of decision making. For new organizations, it also helps create a culture of quality with clearly established expectations. Perhaps most importantly, it provides a baseline when entering the design control process ensuring credible supporting data/evidence and prototype design documentation. For manufacturers designing products for the US markets, they should ensure their procedures comply with 21 CFR subpart D, 820.40 – Document Controls and align with ISO 13485:2016 §4.2.4 Document Control.
Recommendations
We have several recommendations stemming from the introduction of document/version controls:
1) Establishing documentation and test reports describing and assessing a prototype can be completed quickly early in the TFP. Once the team begins approaching the end of the TFP, and begin transitioning into the design control process, it is crucial the team implements and strictly adheres to more formal document and version control procedures.
2) The priority during TFP should be first ensuring all material used for generating evidence is of the utmost quality, and adequate procedures are in place to guarantee that (with the help of supplier controls). As the design matures during TFP, the teams’ priorities can then shift to establishing document/version controls that ensure documentation is properly reviewed, accurate, controlled and aligns with the current design version. Ultimately this means a shift from complying only with purchasing controls to both purchasing and document/version controls.
3) Documenting changes is also a very important element of document controls, but during the TFP there is an opportunity to maximize efficiency. Ensuring design changes and versions are tracked, along with the reason for the change and any relevant supporting data is sufficient. Later in the TFP as the design matures, it is essential to capture document and design changes and the reason for those changes in more detail, in compliance with a formalized process, to ensure information about the design is carried forward into the design control process.
At this point we have complete history of the design iteration during TFP, and we have formal documentation of the final design ready for use in design control. In our next article we will be discussing the completion of the TFP, and the transition into the design control process along with our strategies and recommendations for completing this transition.

Comentarios